WORDS LIM TECK CHOON
 FEATURED EXPERT FEATURED EXPERTASSOCIATE PROFESSOR DR TAN WOOI CHIANG Consultant Dermatologist Gleneagles Hospital Penang and President of the Dermatological Society of Malaysia |
3 KEY FACTS ABOUT ATOPIC DERMATITIS
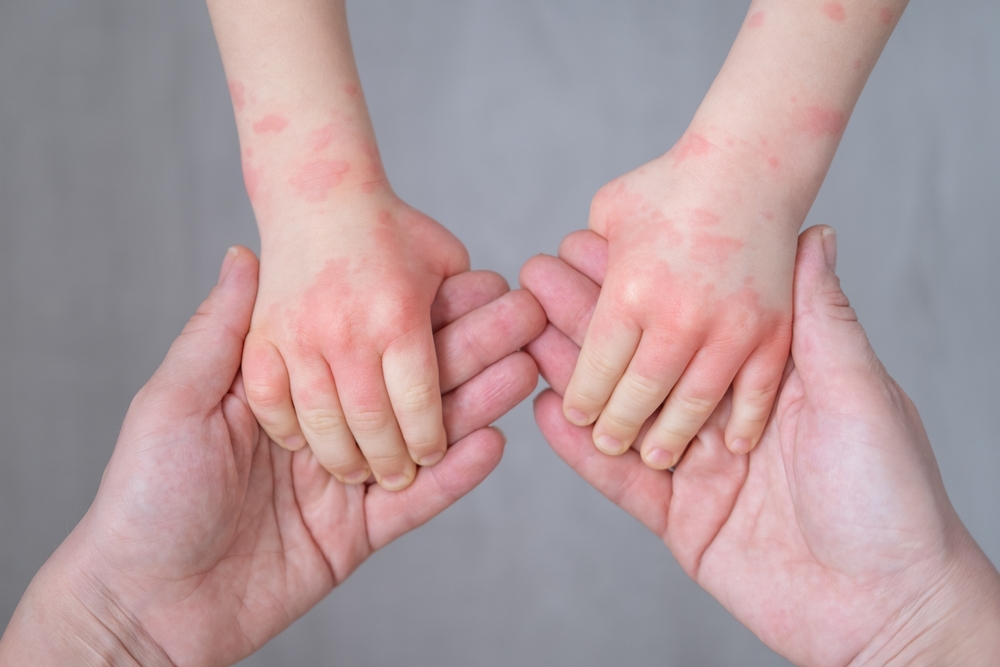
- Atopic dermatitis (AD), commonly known as eczema, is a chronic inflammatory skin condition characterized by dry, itchy, and inflamed skin.
- It typically begins in early childhood, although it can affect individuals at any age.
- The exact cause of AD is not fully understood. “The underlying cause can be attributed to inflammation beneath the skin,” says Dr Tan Wooi Chiang.
- Other possible factors include genetic factors, immune system dysfunction, and environmental triggers that compromise the skin barrier, leading to excessive dryness and susceptibility to infections.
| “Eczema symptoms manifest differently across individuals, exhibiting variations contingent on factors such as person’s age, skin tone, and the severity of the eczema. Physical symptoms aside, patients may also be impacted emotionally, socially, or psychologically,” says Dr Tan Wooi Chiang. |
TO DATE, THERE IS NO CURE FOR AD
However, it can be managed using strategies such as:
- Avoiding triggers that can lead to flare-ups (see below)
- Maintaining a proper skincare routine
- Utilizing medications such as topical corticosteroids to improve symptoms
LIVING WITH ATOPIC DERMATITIS CAN BE CHALLENGING
- AD is marked by periods of flare-ups and remissions, with symptoms often made worse by scratching.
- This need to scratch is due to severe itching that is frequently experienced during flare-ups. The itching can disrupt sleep and daily activities, affecting one’s overall quality of life
- However, scratching can result in further skin damage and increased risk of infection.
- Many people with AD also have from other existing health conditions such as asthma and allergic rhinitis. These health conditions can complicate AD treatment and management.
A NEW TREATMENT FOR MILD TO MODERATE AD
Staquis is a first-in-class steroid-free topical PDE4 inhibitor that can be applied to the skin to treat mild to moderate eczema in patients 3 months of age and older.
PDE4 inhibitors are anti-inflammatory agents that target an immune system enzyme called phosphodiesterase 4 (PDE4), which promotes inflammatory cytokine production and responses.
Staquis works on and below the skin to treat eczema by inhibiting immune system enzyme called phosphodiesterase 4 (PDE4), which promotes inflammatory cytokine production and responses. It also locks in moisture to soften the skin.
Evidence of Efficacy
- Pivotal Phase 3 studies involving individuals 2 years of age and above demonstrated that significantly more patients using Staquis twice a day achieved clear or almost clear skin scores in 29 days.
- The most common adverse reactions were pain at the application site, such as stinging or burning, which was temporary and resolved on its own.
A NEW TREATMENT FOR MODERATE TO SEVERE AD
Cibinqo is a once-a-day oral pill for individuals aged 12 years and older with moderate to severe atopic dermatitis.
It lessens itching and inflammation of the skin by reducing the activity of Janus kinase enzymes, which are thought to contribute to skin inflammation.
Evidence of Efficacy
- Across several randomized, placebo-controlled, Phase 3 trials and an on-going long-term open-label extension trial, Cibinqo demonstrated a consistent safety profile and improvements in skin clearance, extent, and severity of disease.
- A higher proportion of patients treated with Cibinqo in two monotherapy trials achieved improvement in itching at week 12 compared to placebo.
References:
- Pfizer. (2024, November 5). Pfizer introduces two novel therapies to treat patients across the
atopic dermatitis disease spectrum [Press release, KKLIU 3115 / EXP 24-04-2025]. - Reich, K., Silverberg, J. I., Papp, K. A., et al. (2023). Abrocitinib efficacy and safety in patients with moderate-to-severe atopic dermatitis: Results from phase 3 studies, including the long-term extension JADE EXTEND study. Journal of the European Academy of Dermatology and Venereology, 37(10), 2056-2066. https://doi.org/10.1111/jdv.12345
Correction published in Journal of the European Academy of Dermatology and Venereology, 37(12), 2608-2609. - Simpson, E. L., Sinclair, R., Forman, S., et al. (2020). Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. The Lancet, 396(10246), 255-266. https://doi.org/10.1016/S0140-6736(20)31209-1
- Silverberg, J. I., Simpson, E. L., Thyssen, J. P., et al. (2020). Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA dermatology, 156(8), 863-873. https://doi.org/10.1001/jamadermatol.2020.0365